Catalysis
Catalysis in Metal-Organic Frameworks
Oxidation of ethane to ethanol in a Fe2(dobdc) metal-organic framework.[1]
The active sites of metal-organic framework catalysts are not only well-defined and tunable, but also site-isolated. As such, catalytic intermediates that are prone to deactivation by dimerization, such as terminal metal-oxo or metal-dioxygen species, should be much more stable within a rigid framework support. As a result, the metal-organic framework Fe2(dobdc) and its mixed Fe/Mg analogues can activate the strong C–H bonds in ethane using N2O as the terminal oxidant, producing ethanol with high selectivity.
A promising approach to developing highly tunable metal-organic framework catalysts is to synthesize frameworks with open chelating sites. In analogy to how molecular catalysts are tailored by choice of ligand and metal source, we envisioned that a metal-organic framework with chelating linkers could be used as a platform to synthesize catalysts for a variety of reactions through judicious choice of chelating linker and metal source used in post-synthetic metalation.
Molecular Electrocatalysis
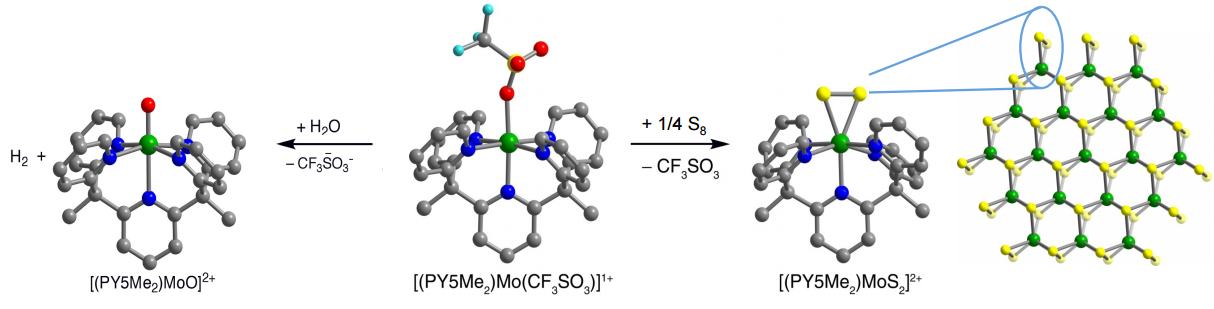
We have also explored catalysis in molecular systems, focusing on transition metal complexes supported by the ligand PY5Me2. This series of molecules has achieved H2 evolution from water, and more recently, CO2 reduction. The complex [(PY5Me2)Mo(CF3SO3)]+ reacts with water to form [(PY5Me2)MoO]2+ with the concomitant evolution of H2. More recently, we have demonstrated that the divalent cobalt complex, [(PY5Me2)Co(H2O)]2+, generates H2 at high turnover frequencies and 100% Faradaic efficiency. Future endeavours include investigating other PY5Me2 complexes, especially those containing first row transition metals; synthesizing new PY5Me2 derivatives and pentadentate ligand scaffolds to tailor the electronic properties of these complexes; and developing systems that demonstrate interesting reactivity in regard to substrates such as H2O, H2, O2, N2, CO2, and CH4.