Conductivity
Overview
The Long Group is actively developing new porous materials capable of bulk charge transport via ions and electrons. In addition to elucidating the design of highly conductive porous conductors we are also interested in pursing possible applications such as: solid electrolytes, electrode materials, and chemical sensors.
Metal-Organic Framework Based Ion Conductors
The structural diversity and crystallinity of metal-organic frameworks makes these materials ideal candidates for the elucidation of ion solvation and transport in nanoporous materials. In particular, we are interested in the synthesis of new materials that conduct ions that are difficult or impossible to conduct through traditional solids.
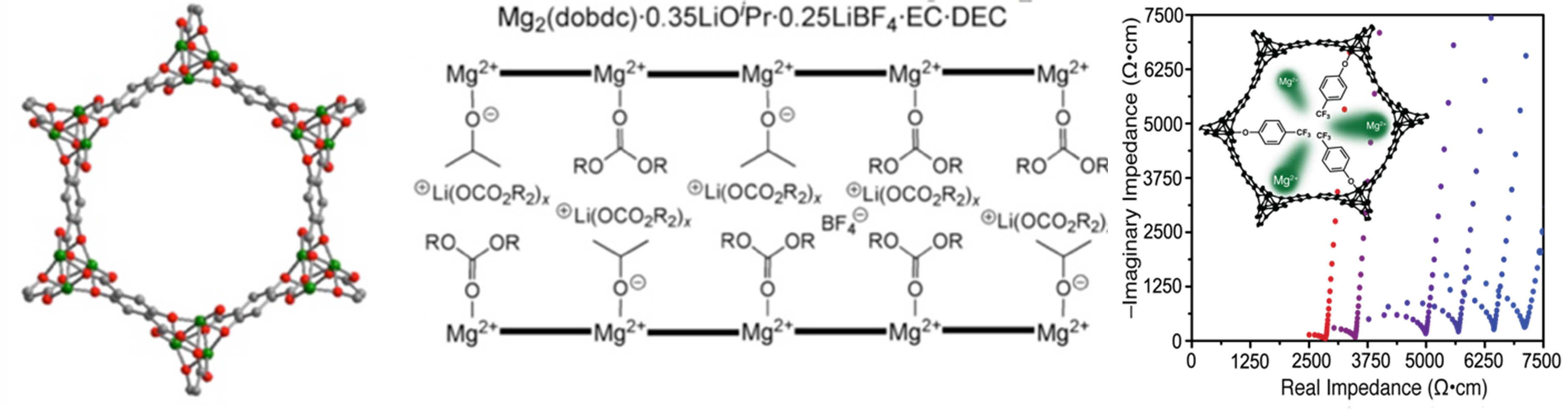
Structure of electrolytes based on the framework Mg2(dobdc) and AC impedance spectra demonstrating bulk conductivity of magnesium electrolytes.
By systematic post-synthetic modification of metal-organic frameworks we have been able to control the ionic conductivity in the parent frameworks Mg2(dobdc) and Mg2(dobpdc) over five orders of magnitude and to obtain ionic bulk conductivities greater than 0.1 mS cm–1 for both lithium ion[1] and magnesium ion[2] conductors. Through this process, our group discovered the only crystalline material to conduct magnesium ions at room temperature.
Single Ion Conducting Porous Aromatic Frameworks
In pursuit of superior electrochemical stability and ion selectivity, we have identified an interesting class of amorphous materials related to MOFs called porous aromatic frameworks. By exchanging the traditionally carbon nodes with anionic tetraarylborates, new lithium ion conductors[3] were synthesized. These materials were found to be selective for the conduction of lithium ions (t+ = 0.93). Importantly, because the material was impregnated with lithium via post-synthetic ion exchange, we expect that the synthesis can be adapted to incorporate other cations as well. The design of single-ion conductors of magnesium and other multivalent ions are underway.
Electron Conducting Metal-Organic Frameworks
Metal-organic frameworks are remarkable materials because of their extremely high surface areas, diverse surface chemistries, and long range atomic ordering. The prospects for engendering long-range electronic communication—manifesting in features such as charge mobility and magnetic ordering—include promising routes to new battery electrodes, electrochemical sensors, bulk magnets and magnetoelectric materials. However, crystals of high porosity (including most metal-organic frameworks) are primarily electronically insulating ionic solids, lacking long-range electronic communication.
Nonetheless, the structural diversity of porous hybrid materials like metal-organic frameworks allows the synthetic chemist to precisely target and manipulate metal-ligand interactions amenable to periodic electronic coupling. We are interested in the synthesis and characterization of conductive metal-organic frameworks, elucidation of underlying transport mechanisms by physical methods, and demonstration of emergent applications.
Permanently Porous Conductive Metal-Organic Frameworks
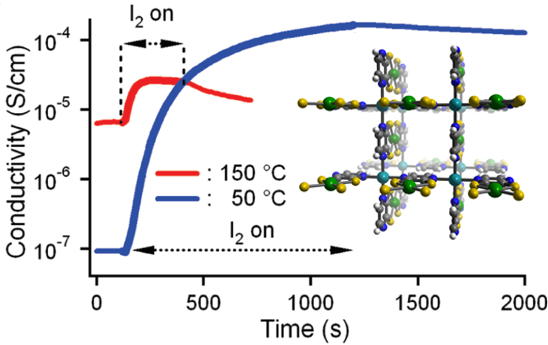
Porous conductors with tunable surface chemistries are of technological interest for chemresistive/ electrochemical sensing, selective gas sorption, and voltage gated adsorption. We have previously reported a metal-organic framework composed of the redox-active subunit Ni(pdt)22– connected in three-dimensions by Cu2+ ions to form Cu[Ni(pdt)2][4]. Upon desolvation of the as synthesized phase, this material displays simultaneous permanent porosity and bulk charge transport. Following exposure of the framework to I2 vapor, pressed pellet conductivities as high as 0.1 mS/cm were obtained.
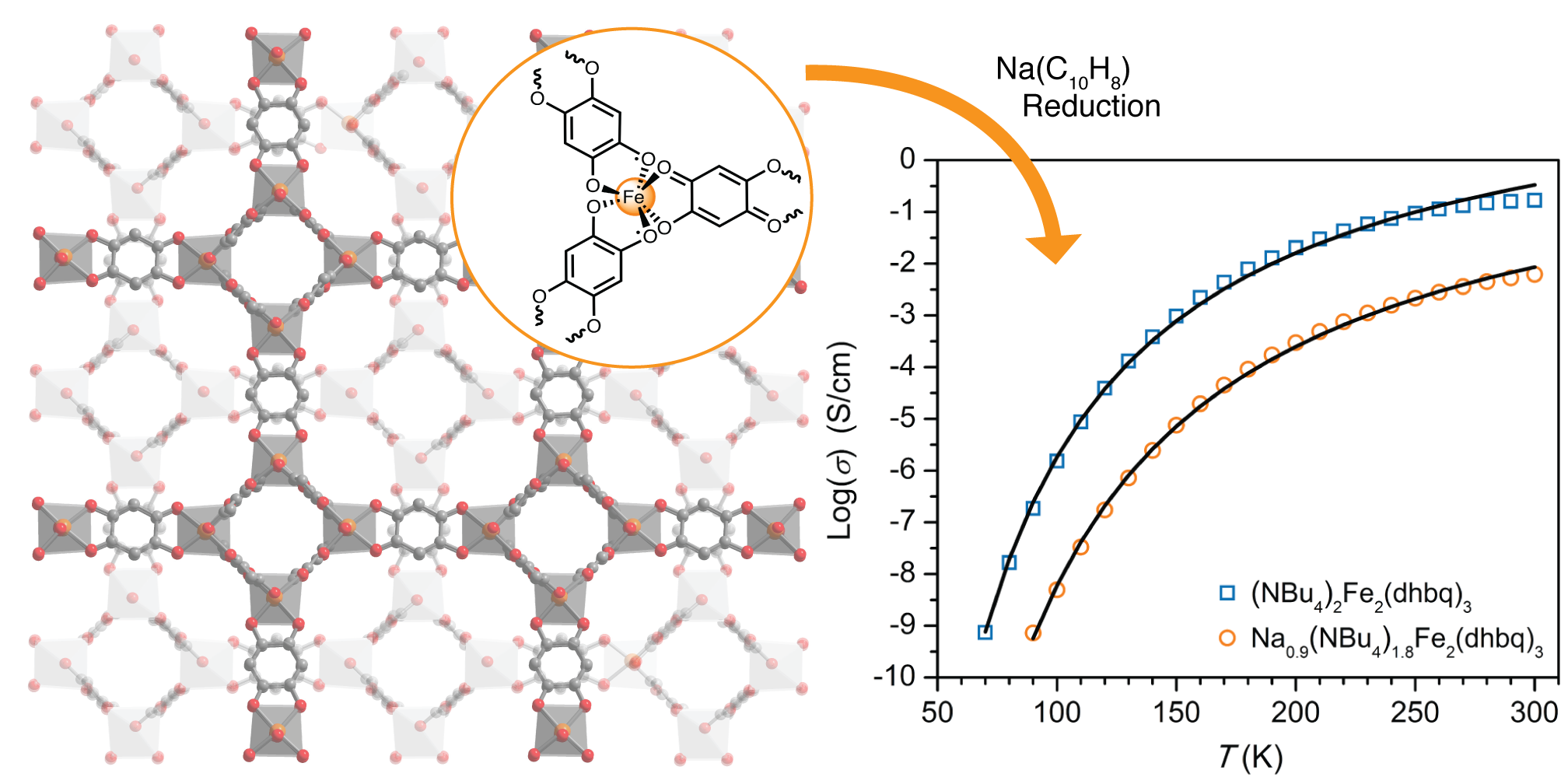
Charge Transport via Ligand Centered Mixed-Valence
Metal centers that participate in strong π-d coupling with ligand-based orbitals can behave as efficient bridges for electron transfer between organic radicals. We have recently reported the single-step formation of a ferric-semiquinoid framework[5] that displays Robin-Day Class IIB organic mixed-valence with a 1:3 ratio of quinoid: semiquinoid ligands. The as-synthesized material has a conductivity of 0.16 S/cm at room temperature, one the highest values reported for a metal-organic framework. We are actively pursuing more conductive frameworks by adapting this approach to a diverse set of metals, ligands and framework topologies.