Molecular Magnetism
Mono- and Multinuclear Single-Molecule Magnets
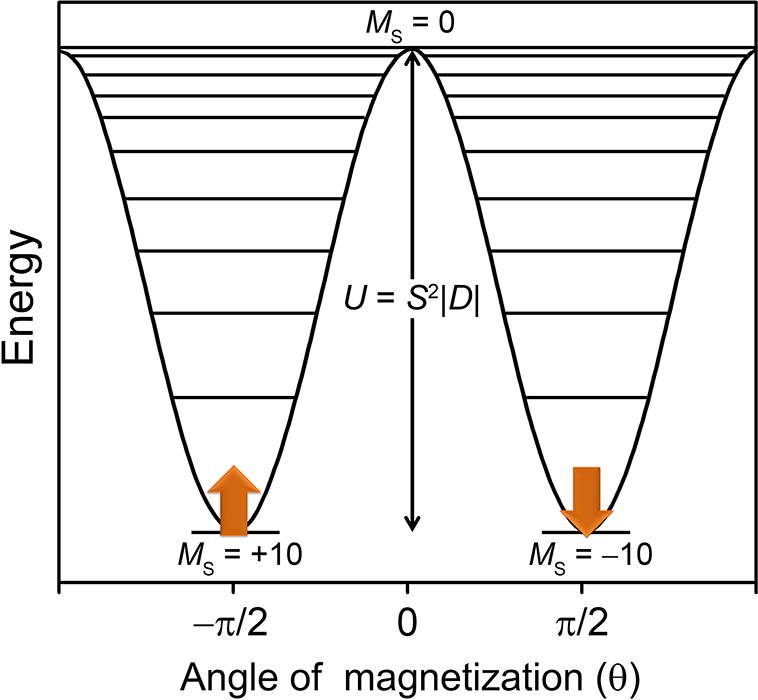
Single-molecule magnets are a class of materials that retain their magnetization at low temperatures, much like bulk magnets. This property arises from a bistable magnetic ground state, wherein the “up” or “down” orientations of the magnetization are separated by an energy barrier, U. These molecules demonstrate such phenomena as magnetic hysteresis, and are of potential interest for applications in spin-based electronics and quantum computing. Currently, such systems only demonstrate operating temperatures below 20 K, however. One of the main thrusts of single-molecule magnetism research is therefore to design systems with much higher barriers and hysteresis temperatures.
Systems incorporating the lanthanide ions remain one of the most promising avenues, due to the high magnetic moments of these metals and the large, unquenched orbital angular momentum of the magnetic 4f orbitals. Recently, we demonstrated that the molecule Er[(COT)2]– exhibits magnetic hysteresis to 10 K, representing one of the highest temperatures for a mononuclear lanthanide material. Here, the ligand field symmetry is crucial to the magnetic properties, and we are continuing to explore the impact of ligand field symmetry and donor strength on U.
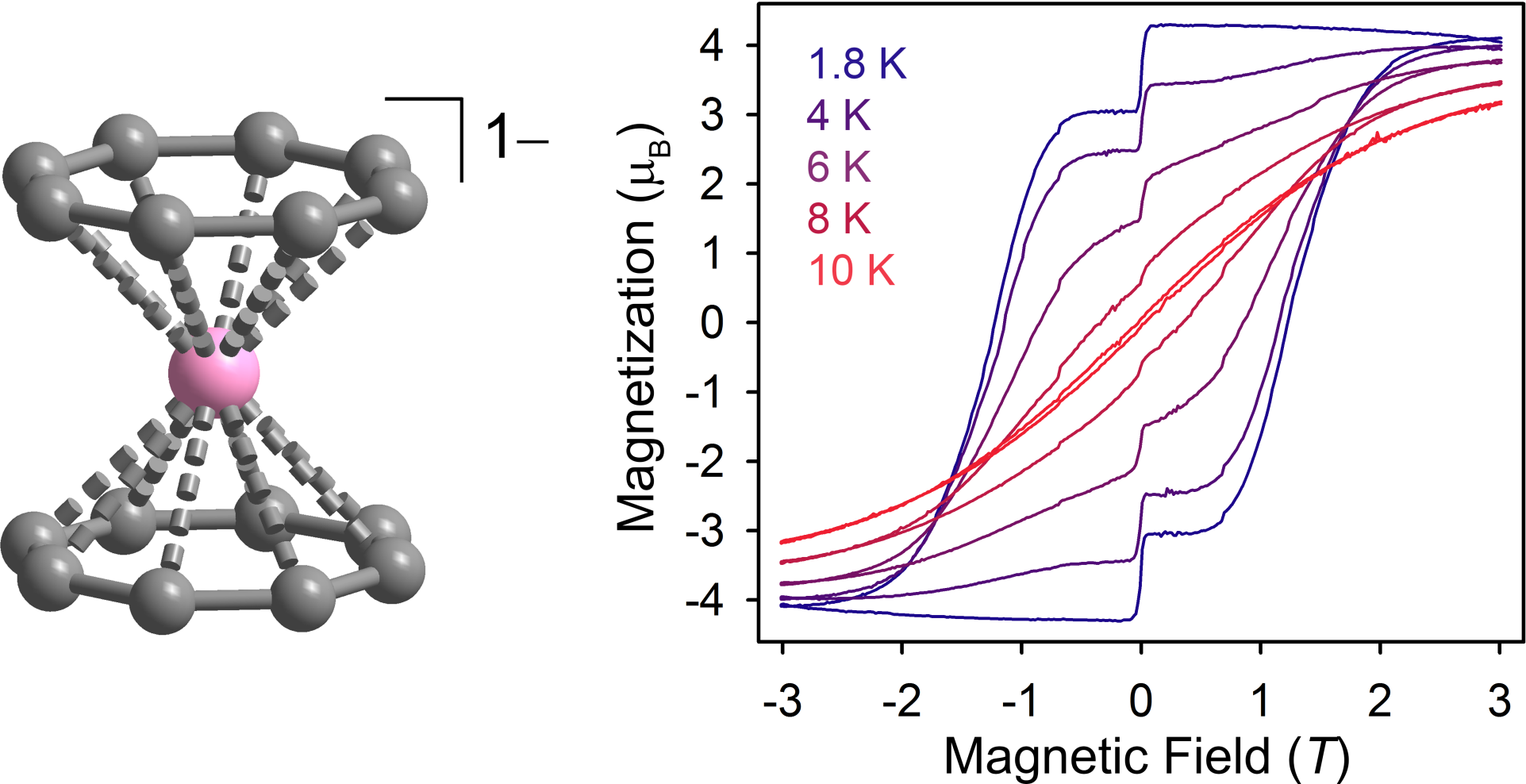
X-ray structure and magnetic hysteresis for Er[(COT)2]–.[1]
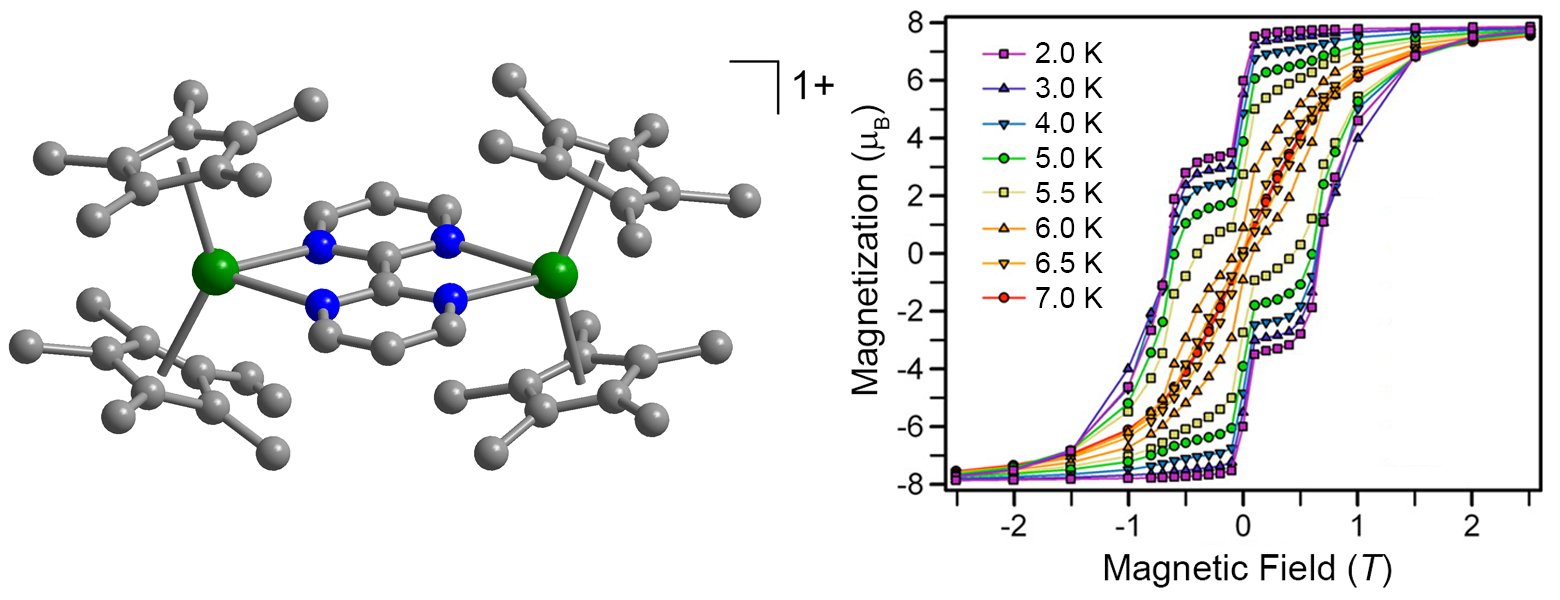
X-ray structure and magnetic hysteresis for [(Cp*2Dy)2(μ-bpym•)]+.[2]
Another strategy is to couple two magnetic centers. We have recently demonstrated that coupling through a radical ligand is particularly effective in the molecule [(Cp*2Dy)2(μ-bpym•)]+, wherein the lanthanide ions are bridged by a bipyrimidine radical. We are currently pursuing higher nuclearity structures such as radicalbridged chains and tri- and tetranuclear species.
Recently, we have also shown that mononuclear systems incorporating cheap, earth-abundant metals such as iron can behave as single-molecule magnets. We have accomplished this through the design of low-coordinate complexes that possess enhanced preference for orientation of the magnetic moment, leading to large relaxation barriers U and magnetic hysteresis, as seen for the mononuclear FeI species [Fe(C(SiMe3)3)2]-.
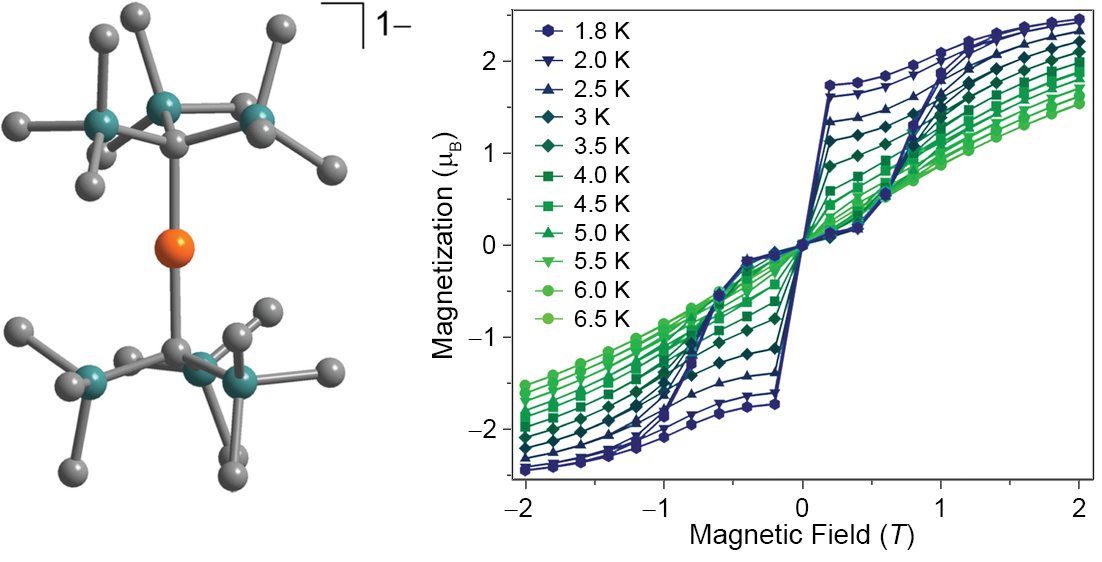
X-ray structure and magnetic hysteresis for [Fe(C(SiMe3)3)2]-.[3]