Metal-Organic Frameworks
Gas Storage and Separation
We are developing metal-organic frameworks for room temperature hydrogen and methane storage for applications in automotive transportation. Both of these gases are cleaner burning than gasoline but cannot be stored at sufficient densities at room temperature for use as fuels. For this to be feasible, the density of strong binding sites for both of these gases must be increased over currently known adsorbents. Metal-organic frameworks offer high porosity and control of the binding sites within the framework pores; by selectively modifying frameworks and exploring new frameworks, we are discovering materials that strongly interact with and store both of these gases at high densities.
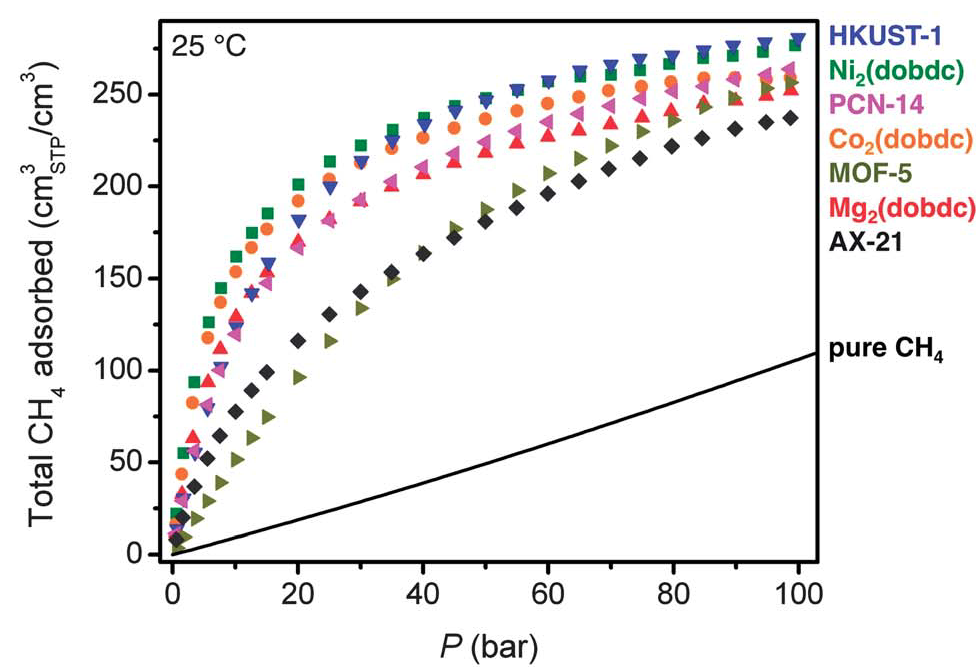
Methane isotherms for a variety of metal-organic frameworks.[1]
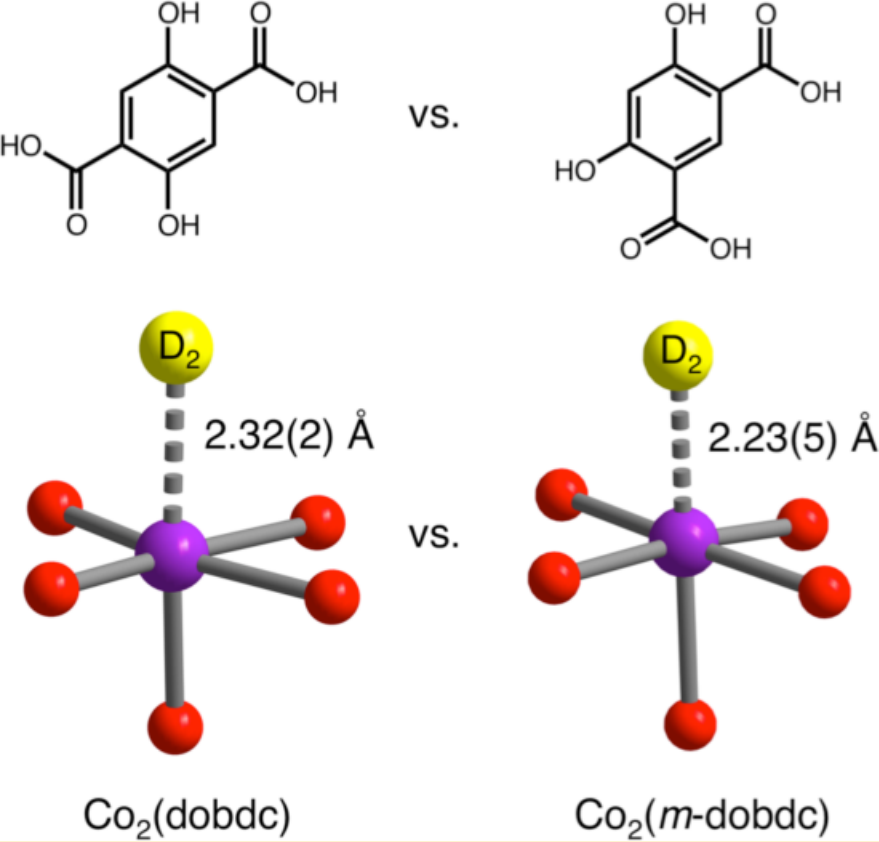
D2 binding onto the coordinatively unsaturated metal site of Co2(dobdc) and Co2(m-dobdc). Meta functionality in the linker causes stronger binding. [2]

CO2 adsorption isotherms in mmen-Mg2(dobpdc) at various temperatures. [3]
A promising strategy for mitigating CO2 emissions is to remove CO2 directly from the flue gas of existing coal-fired power plants. An ideal material for this separation will selectively bind CO2 in the presence of N2 and H2O, and release it under mild regeneration conditions.
Metal-organic frameworks with diamine functionality, such as mmen-Mg2(dobpdc), exhibit a co-operative insertion mechanism leading to high CO2/N2 selectivities and uptake capacities in the presence of H2O. The energy costs associated with the separation of light hydrocarbons could potentially be lowered through the development of selective solid adsorbents.
We have recently synthesized a metal-organic framework, Fe2(dobdc), featuring channels lined with a high concentration of soft Fe2+ cation sites, which exhibits excellent characteristics for the separation of the ethylene/ethane and propylene/propane mixtures at 318 K. The material can be expected to exhibit higher selectivities and capacities compared to other known adsorbents for: the fractionation of ethane/ethane/ethylene/acetylene mixtures, removing acetylene impurities from ethylene, and membrane-based olefin/paraffin separations. Methane, ethane, ethylene, and acetylene breakthrough curves were calculated for an equimolar mixture of the gases flowing through a bed of Fe2(dobdc). The different interactions that each of these gas molecules have with the Fe2(dobdc) open coordination sites determines how well they can be separated and their elution times in a flow-through setup. By using just three packed beds of Fe2(dobdc), the successful separation of a mixture of these gases can be obtained.
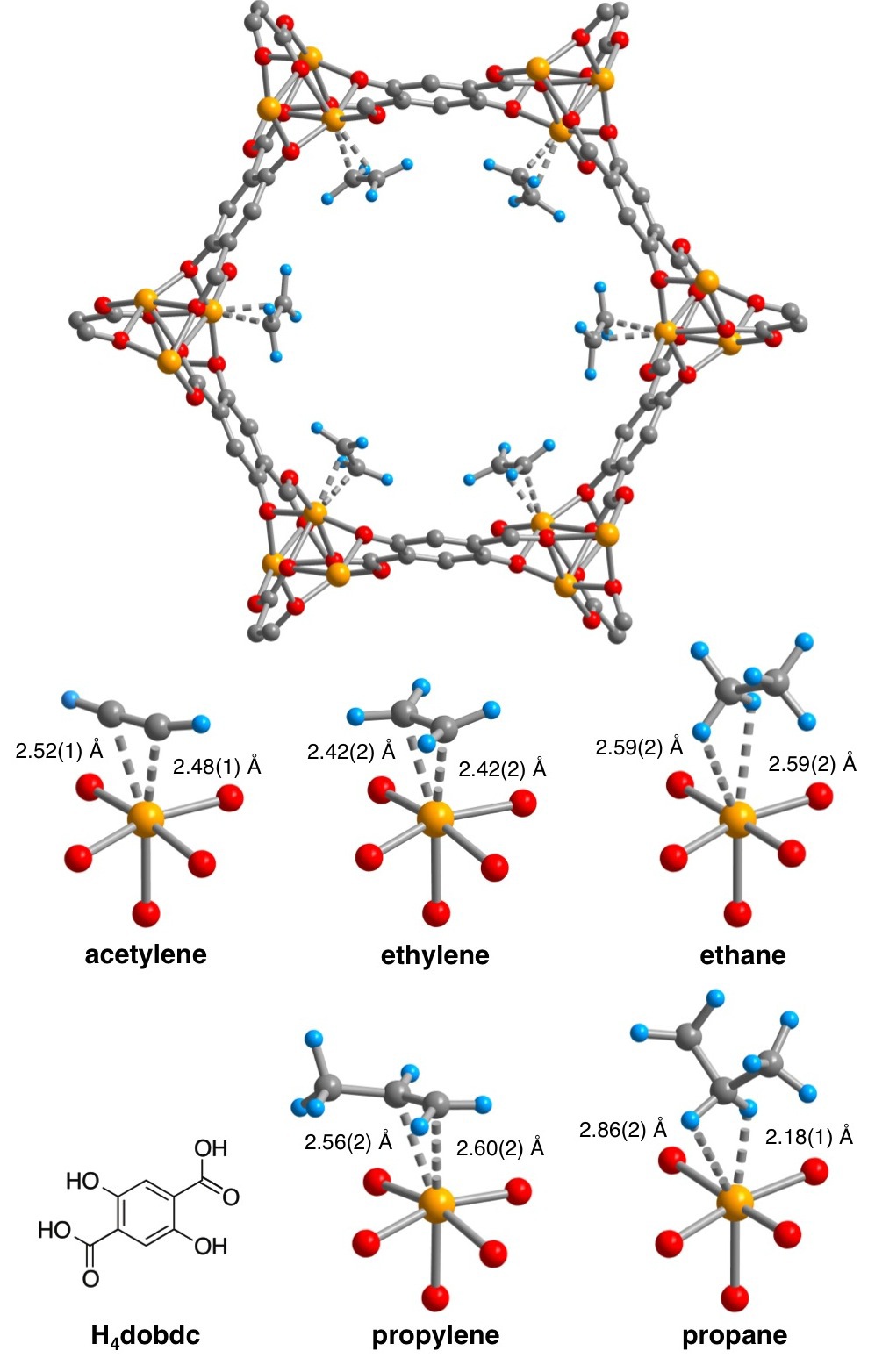
Structures of hydrocarbons bound to the open coordination sites of Fe2(dobdc). [4]
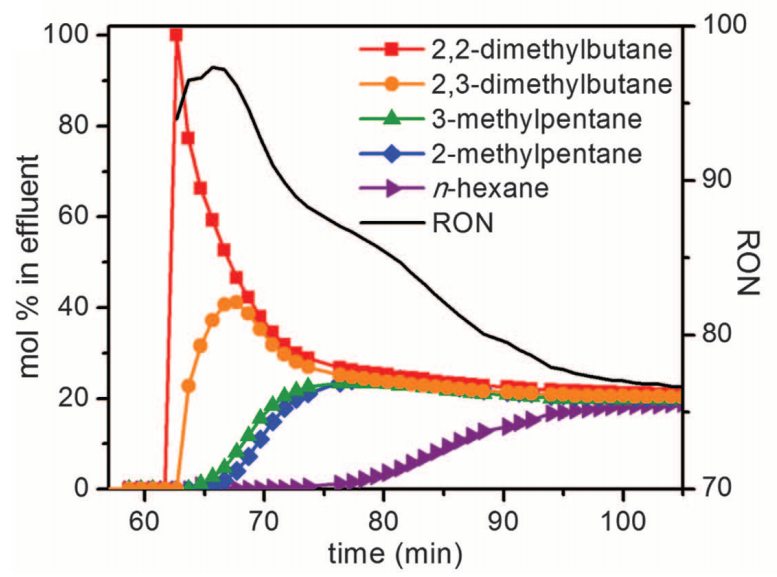
Experimental breakthrough curves for various hexane isomers in the Fe2(BDP)3 framework. [5]
The separation of the various isomers of hexane is critically important to the production of gasoline. Higher octane number isomers are more industrially desirable, yet this separation is difficult due to low reactivity and polarizability differences between the isomers.
Separation of these isomers from a mixture of hexanes was achieved in the Fe2(BDP)3 framework by leveraging different interaction with the acute angles of the pores in this framework. More linear, lower octane isomers have stronger interactions and are retained, leading to separation of the branched, higher-octane isomers from less industrially-valuable isomers.